Clinical Trials
This section provides information about the Clinical Trials source ,
listing each field available for Clinical Trials, with details
about the field value type, including whether it may be used as a facet
and whether it is a multi-value field. The table also contains information about whether
the field may be used in the where section and whether the
field the field may be used in the entity fields filter.
The page also lists the fields included in each fieldset available
for Clinical Trials, and each aggregation indicator available.
Note
By default, Clinical Trials is sorted by the relevance, unless specified differently using Sorting Results option.
Clinical Trials Fields
Fields are used to perform searches on the source they belong to. Note: the emptiness filters can be used on any field, irrespectively of whether filtering / faceting is enabled on them.
Field |
Type |
Multi? |
Filter? |
Facet? |
Description |
|---|---|---|---|---|---|
|
Literal: string |
Abstract or description of the clinical trial. |
|||
|
Literal: string |
✓ |
Acronym of the clinical trial. |
||
|
Literal: integer |
✓ |
✓ |
✓ |
List of active years for a clinical trial. |
|
Literal: float |
✓ |
Altmetric Attention Score. |
||
|
Literal: string |
✓ |
✓ |
Dimensions IDs of the grants associated to the clinical trial (see also: Clinical Trials Links section). |
|
|
Literal: string |
✓ |
Brief title of the clinical trial. |
||
|
Entity: categories |
✓ |
✓ |
✓ |
|
|
Entity: categories |
✓ |
✓ |
✓ |
ANZSRC Fields of Research classification (alias for most recent version). |
|
Entity: categories |
✓ |
✓ |
✓ |
|
|
Entity: categories |
✓ |
✓ |
✓ |
|
|
Entity: categories |
✓ |
✓ |
✓ |
|
|
Entity: categories |
✓ |
✓ |
✓ |
|
|
Entity: categories |
✓ |
✓ |
✓ |
|
|
Entity: categories |
✓ |
✓ |
✓ |
|
|
Entity: categories |
✓ |
✓ |
✓ |
|
|
Literal: string |
✓ |
✓ |
List of medical conditions names, e.g. ‘Breast cancer’ or ‘Obesity’. |
|
|
Literal: date |
✓ |
Date when the record was inserted into Dimensions (note: this field does not support exact match on the data, only range filters e.g. <= or `>=’). |
||
|
Literal: string |
Link pointing to the Dimensions web application |
|||
|
Literal: date |
✓ |
End of date of the clinical trial. |
||
|
Entity: countries |
✓ |
✓ |
✓ |
The country group the funding organisations. |
|
Entity: organizations |
✓ |
✓ |
✓ |
GRID funding organisations that are involved with the clinical trial. |
|
Literal: string |
✓ |
The gender of the clinical trial subjects e.g. ‘Male’, ‘Female’ or ‘All’. |
||
|
Literal: string |
✓ |
Dimensions clinical trial ID |
||
|
Literal: json |
✓ |
✓ |
Structured JSON object containing information about the clinical trial’s interventions according to the research plan or protocol created by the investigators. [1] |
|
|
Literal: json |
✓ |
✓ |
Additional details about investigators, including affiliations and roles. |
|
|
Literal: string |
Original URL for the clinical trial. |
|||
|
Literal: string |
✓ |
✓ |
✓ |
Medical Subject Heading terms as used in PubMed. |
|
Literal: string |
✓ |
✓ |
Status of the trial as provided by the registry. |
|
|
Literal: string |
✓ |
Phase of the clinical trial, as a string. |
||
|
Literal: string |
✓ |
✓ |
Publications mentioned in clinical trials (excluding resulting publications), as a list of Dimensions identifiers. |
|
|
Entity: publication_links |
✓ |
✓ |
✓ |
Publications mentioned in clinical trials (excluding resulting publications), as a structured entity. |
|
Literal: string |
✓ |
The platform where the clinical trial has been registered, e.g. ‘ClinicalTrials.gov’ or ‘EU-CTR’. |
||
|
Entity: organizations |
✓ |
✓ |
✓ |
GRID organizations involved, e.g. as sponsors or collaborators. |
|
Entity: researchers |
✓ |
✓ |
✓ |
Dimensions researchers IDs associated to the clinical trial. |
|
Literal: float |
✓ |
For full-text queries, the relevance score is a numerical value assigned to a document that indicates how relevant that document is for a given query. Note: the score is query-specific i.e. it cannot be used to compare documents across queries. |
||
|
Literal: date |
✓ |
Start of date of the clinical trial. |
||
|
Literal: string |
✓ |
✓ |
✓ |
The study arms. |
|
Literal: string |
✓ |
✓ |
✓ |
The study design methods |
|
Literal: string |
The eligibility criteria. |
|||
|
Literal: string |
✓ |
✓ |
The maximum age. |
|
|
Literal: string |
✓ |
✓ |
The minimum age. |
|
|
Literal: json |
✓ |
✓ |
The study outcomes. |
|
|
Literal: integer |
✓ |
✓ |
The number of participants. |
|
|
Literal: string |
✓ |
✓ |
The type of study. |
|
|
Literal: string |
The title of the clinical trial. |
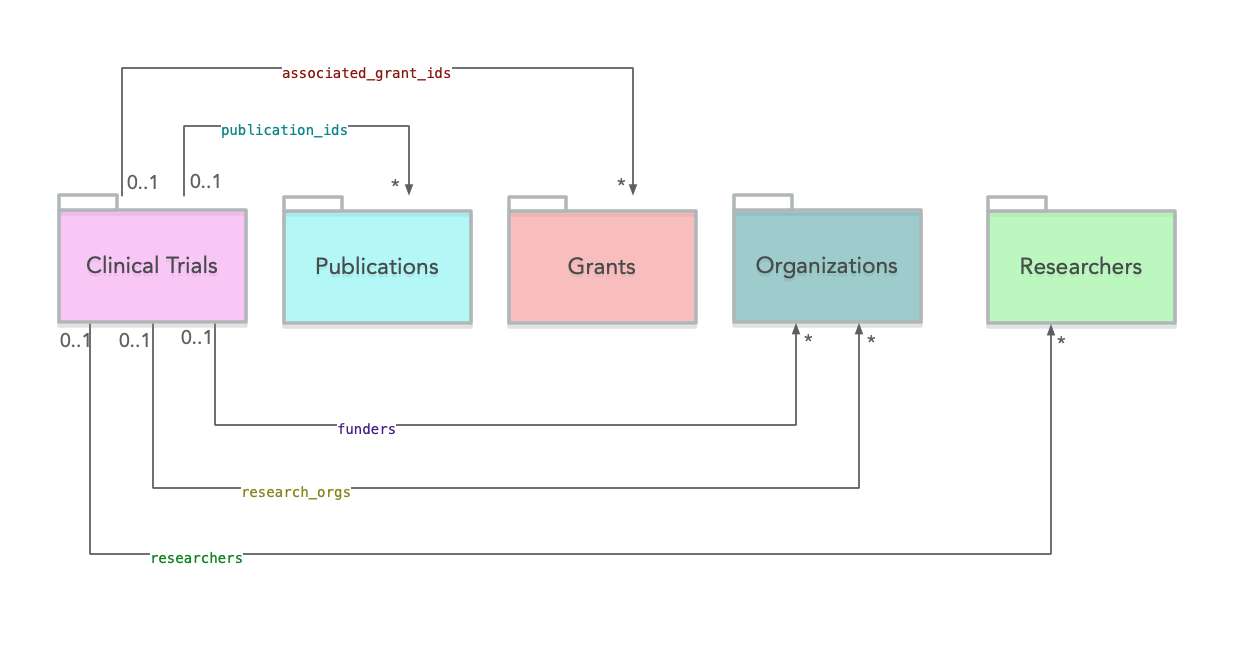
Clinical Trials Links
The diagram below provides an overview of the outgoing links from Clinical Trials to other sources.
Clinical Trials Fieldsets
Fieldsets are used to specify fields groups in the context of a return statement.
Fieldset |
Fields |
|---|---|
|
|
|
|
|
|
|
|
Clinical Trials Indicators
Indicators are used to aggregate results in the context of a facet query.
Indicator |
Description |
|---|---|
|
Total count |
Clinical Trials Search Indexes
Search indexes are used to perform full-text searches on the source they belong to. The default search index, which is used if none other is specified, is full_data.
Search index |
Supports multi-index search |
Description |
|---|---|---|
|
✓ |
Search for keywords in title only. |
|
✓ |
The keywords will be searched for in the title and description/summary. |
|
✓ |
Search within the raw names of the involved research organizations. Note: Depending on the selected content type the organizations can have different roles: Publications (author affiliation), Grants (funded organization/investigator affiliations), Patents (current and original assignee), Clinical trials (sponsor and collaborating organization). Boolean nesting to process phrases or words in a specified order is not supported. |
|
Search for keywords in the investigators fields of a clinical trial. |
|
|
✓ |
The keyword will be searched for in all data fields that are found for that document. In cases where the full text is indexed in the background this will also be searched, even though the full text may not be available to see within Dimensions itself. This will mean that many results that are returned may not include mention of the searched keyword on the document page, but are returned in the search because the keyword can be found in the full text which cannot be read. |
Clinical Trials Deprecations
This section lists fields and search fields that have been deprecated and should not be used.
Deprecated Fields
Field |
Deprecation Note |
|---|---|
|
Deprecated in favor of: |
Partially Deprecated Fields
Clinical Trials source has no partially deprecated fields.
Deprecated Search Indexes
Clinical Trials source has no deprecated search indexes.