Clinical Trials
This page contains information about the Clinical Trials data source in the Dimensions BigQuery project.
See also
Dimensions DSL API Documentation for this type is also available from here.
Clinical Trials Schema
Field |
Type |
Description |
Example |
|---|---|---|---|
|
Literal: String |
Abstract or description of the clinical trial. |
"Adolescent young carers (AYCs) are young people aged 15-17 years old, who take on significant or substantial caring tasks and assume a level of responsibility that would usually be associated with an adult. In Europe, the estimated prevalence rate of YCs is around 4-8%.Taking on care responsibilities so early in life may have considerable negative consequences for YCs' mental and physical health and psychosocial development. Psychosocial interventions to support YC worldwide are generally quite limited. The H2020 Me-We project (Psychosocial Support for Promoting Mental Health and Well-being among Adolescent Young Carers in Europe) aims to develop an innovative framework of primary prevention interventions for adolescent YCs (AYCs) aged 15-17 to be tested in six European countries (Italy, Netherlands, Slovenia, Sweden, Switzerland, United Kingdom). The theoretical framework chosen for the intervention is the DNA-V Model. The DNA-V model is a psychological intervention, addressed to adolescents and young people, used in educational and clinical settings. This model has its roots in the contextual and functional science and it is based on Acceptance and Commitment Therapy, a third-generation cognitive-behavioural therapy. The intervention programme designed for the ME-WE project builds on the DNA-V model but it was adapted to fit the specific needs of adolescent young carers (AYCs) and the goals of the ME-WE project. The study aim is to evaluate the efficacy of DNA-V based program for AYCs (so-called ME-WE intervention), using a cluster-randomized controlled trial (C-RCT) design. The evaluation of the intervention will be carried out using as primary outcome variables: Psychological flexibility; Mindfulness skills; Resilience; Subjective mental health; Quality of life; Subjective health complaints; Caring-related quality of life; Cognitive and emotional impact of caring and Social support. As secondary outcome variables will be included Self-reported school, training or work experience, performance, and attendance.\n\nDetailed Description\nAdolescent young carers (AYCs) are young people aged 15-17 years old, who take on significant or substantial caring tasks and assume a level of responsibility that would usually be associated with an adult. Often on a regular basis, they look after family member(s) with a disability, chronic physical and/or mental health condition or substance use issue and/or problems related to old age, who require support or supervision. In Europe, the estimated prevalence rate of YCs is around 4-8%. Taking on care responsibilities so early in life may have considerable negative consequences for YCs' mental and physical health and psychosocial development. Furthermore, YCs likely face difficulties in education that negatively impact their future employability and socio-economic status and experience constraints in finding and maintaining employment and pursuing their career aspirations. Psychosocial interventions to support YC worldwide are generally quite limited. In order to prevent the entrenched level of caring that results in significant and long-term effects on YCs' well-being and hinder transitions to adulthood, it has been suggested that a primary prevention model should be adopted. To prevent adverse mental health, social, and educational outcomes in YCs, building their resilience would be especially important. The H2020 Me-We project (Psychosocial Support for Promoting Mental Health and Well-being among Adolescent Young Carers in Europe) aims to develop an innovative framework of primary prevention interventions for adolescent YCs (AYCs) aged 15-17 to be tested in six European countries (Italy, Netherlands, Slovenia, Sweden, Switzerland, United Kingdom). The theoretical framework chosen for the intervention is the DNA-V Model. The DNA-V model is a psychological intervention, addressed to adolescents and young people, used in educational and clinical settings. This model has its roots in the contextual and functional science and it is based on Acceptance and Commitment Therapy, a third-generation cognitive-behavioural therapy. The intervention programme designed for the ME-WE project builds on the DNA-V model but it was adapted to fit the specific needs of adolescent young carers (AYCs) and the goals of the ME-WE project. The study aim is to evaluate the efficacy of DNA-V-based program for AYCs, called the ME-WE support intervention, using a cluster-randomized controlled trial (C-RCT) design. The evaluation of the intervention will be carried out using as primary outcome variables: Psychological flexibility; Mindfulness skills; Resilience; Subjective mental health; Quality of life; Subjective health complaints; Caring-related quality of life; Cognitive and emotional impact of caring and Social support. As secondary outcome variables Self-reported school, training or work experience, performance, and attendance will be used. Control variable will be caring activities; overall amount of caring and likes and dislikes about caring. Results will be compared of the intervention-group participants relative to the wait-list control-group participants from baseline (pre-intervention) through post-intervention and 3-month follow-up (3MFU). Investigators expect that there will be greater improvements in protective factors targeted by the ME-WE intervention. Thus, it is hypothesized that, compared to the wait-list control group, ME-WE participants will report greater improvements in psychological flexibility, mindfulness, resilience, subjective mental health and quality of life as well as in perceived emotional impact of caring and social support (primary outcomes), and these effects will be maintained at the 3MFU. The impact of ME-WE on self-reported school, training or work experience, performance, and attendance of AYCs (secondary outcomes) will be also explored. Since the intervention will not address these variables directly, we consider them as secondary outcomes."
|
|
Literal: String |
Acronym of the clinical trial. |
"ME-WE"
|
|
Array of < Literal: Integer > |
List of active years for a clinical trial. |
[2019, 2020, 2021]
|
|
Entity: Altmetric |
If available, Altmetric details relating to the clinical trial. |
{"id": "75045719", "score": 128}
|
|
Array of < Literal: String > |
Dimensions IDs of the grants associated to the clinical trial. |
["grant.6443089", "grant.2690176", "grant.6499358"]
|
|
Literal: String |
Brief title of the clinical trial. |
"A Primary Prevention Intervention for the Promotion of Psycho-social Wellbeing in Adolescent Young Carers:"
|
|
Entity: VersionedCategories |
Versioned categorisations. |
{"bra_v1": {"values": ["Public Health"], "full": [{"id": "4003", "value": "Public Health"}]}, "for_v1": {"first_level": {"codes": ["11", "13", "17"], "full": [{"id": "2211", "code": "11", "name": "Medical and Health Sciences"}, {"id": "2213", "code": "13", "name": "Education"}, {"id": "2217", "code": "17", "name": "Psychology and Cognitive Sciences"}]}, "second_level": {"codes": ["1303", "1117", "1701"], "full": [{"id": "3268", "code": "1303", "name": "Specialist Studies In Education"}, {"id": "3177", "code": "1117", "name": "Public Health and Health Services"}, {"id": "3468", "code": "1701", "name": "Psychology"}]}}, "hra_v1": {"values": ["Population & Society"], "full": [{"id": "3903", "value": "Population & Society"}]}, "hrcs_hc_v1": {"values": ["Mental Health"], "full": [{"id": "905", "value": "Mental Health"}]}, "hrcs_rac_v1": {"codes": ["6.6", "7.1"], "full": [{"id": "10606", "code": "6.6", "name": "Psychological and behavioural"}, {"id": "10701", "code": "7.1", "name": "Individual care needs"}]}, "icrp_cso_v1": {"codes": ["6.6", "6.5", "6.3", "6.1", "6.9"], "full": [{"id": "3777", "code": "6.6", "name": "End-of-Life Care"}, {"id": "3776", "code": "6.5", "name": "Education and Communication"}, {"id": "3774", "code": "6.3", "name": "Behavior"}, {"id": "3772", "code": "6.1", "name": "Patient Care and Survivorship Issues"}, {"id": "3780", "code": "6.9", "name": "Resources and Infrastructure Related to Cancer Control, Survivorship, and Outcomes Research"}]}, "icrp_ct_v1": {"values": ["Not Site-Specific Cancer"], "full": [{"id": "3816", "value": "Not Site-Specific Cancer"}]}, "rcdc_v1": {"values": ["Prevention", "Clinical Trials and Supportive Activities", "Behavioral and Social Science", "Mind and Body", "Mental Health", "Clinical Research", "Complementary and Alternative Medicine", "Pediatric"], "full": [{"id": "558", "value": "Prevention"}, {"id": "508", "value": "Clinical Trials and Supportive Activities"}, {"id": "498", "value": "Behavioral and Social Science"}, {"id": "458", "value": "Mind and Body"}, {"id": "380", "value": "Mental Health"}, {"id": "507", "value": "Clinical Research"}, {"id": "430", "value": "Complementary and Alternative Medicine"}, {"id": "547", "value": "Pediatric"}]}, "sdg_v1": null, "uoa_v1": {"codes": ["A03"], "full": [{"id": "30003", "code": "A03", "name": "Allied Health Professions, Dentistry, Nursing and Pharmacy"}]}}
|
|
Entity: SimpleCategorization |
{"values": ["Public Health"], "full": [{"id": "4003", "value": "Public Health"}]}
|
|
|
Entity: ComplexSystematicCategorization |
{"first_level": {"codes": ["11", "13", "17"], "full": [{"id": "2211", "code": "11", "name": "Medical and Health Sciences"}, {"id": "2213", "code": "13", "name": "Education"}, {"id": "2217", "code": "17", "name": "Psychology and Cognitive Sciences"}]}, "second_level": {"codes": ["1303", "1117", "1701"], "full": [{"id": "3268", "code": "1303", "name": "Specialist Studies In Education"}, {"id": "3177", "code": "1117", "name": "Public Health and Health Services"}, {"id": "3468", "code": "1701", "name": "Psychology"}]}}
|
|
|
Entity: SimpleCategorization |
{"values": ["Population & Society"], "full": [{"id": "3903", "value": "Population & Society"}]}
|
|
|
Entity: SimpleCategorization |
{"values": ["Mental Health"], "full": [{"id": "905", "value": "Mental Health"}]}
|
|
|
Entity: SystematicCategorization |
{"codes": ["6.6", "7.1"], "full": [{"id": "10606", "code": "6.6", "name": "Psychological and behavioural"}, {"id": "10701", "code": "7.1", "name": "Individual care needs"}]}
|
|
|
Entity: SystematicCategorization |
{"codes": ["6.6", "6.5", "6.3", "6.1", "6.9"], "full": [{"id": "3777", "code": "6.6", "name": "End-of-Life Care"}, {"id": "3776", "code": "6.5", "name": "Education and Communication"}, {"id": "3774", "code": "6.3", "name": "Behavior"}, {"id": "3772", "code": "6.1", "name": "Patient Care and Survivorship Issues"}, {"id": "3780", "code": "6.9", "name": "Resources and Infrastructure Related to Cancer Control, Survivorship, and Outcomes Research"}]}
|
|
|
Entity: SimpleCategorization |
{"values": ["Not Site-Specific Cancer"], "full": [{"id": "3816", "value": "Not Site-Specific Cancer"}]}
|
|
|
Entity: SimpleCategorization |
{"values": ["Prevention", "Clinical Trials and Supportive Activities", "Behavioral and Social Science", "Mind and Body", "Mental Health", "Clinical Research", "Complementary and Alternative Medicine", "Pediatric"], "full": [{"id": "558", "value": "Prevention"}, {"id": "508", "value": "Clinical Trials and Supportive Activities"}, {"id": "498", "value": "Behavioral and Social Science"}, {"id": "458", "value": "Mind and Body"}, {"id": "380", "value": "Mental Health"}, {"id": "507", "value": "Clinical Research"}, {"id": "430", "value": "Complementary and Alternative Medicine"}, {"id": "547", "value": "Pediatric"}]}
|
|
|
Array of < Entity: Concept > |
Concepts describing the main topics of a clinical trial (note: automatically derived from the publication text using machine learning). |
[{"concept": "type 2 diabetes", "relevance": "0.6613533271499066"}, {"concept": "major adverse cardiovascular events", "relevance": "0.5938189233667946"}, {"concept": "adverse cardiovascular events", "relevance": "0.5781304951906274"}, {"concept": "cardiovascular events", "relevance": "0.5354345934681063"}, {"concept": "cardiovascular risk", "relevance": "0.5347364147008445"}, {"concept": "diabetes", "relevance": "0.4813957362803809"}, {"concept": "dulaglutide", "relevance": "0.42067410295695584"}, {"concept": "patients", "relevance": "0.4159796606977385"}, {"concept": "tirzepatide", "relevance": "0.4110294195515764"}, {"concept": "trials", "relevance": "0.4009629667128156"}, {"concept": "efficacy", "relevance": "0.3967648772262315"}, {"concept": "risk", "relevance": "0.385950719274986"}, {"concept": "safety", "relevance": "0.3671329335686628"}, {"concept": "participants", "relevance": "0.3633964434901934"}, {"concept": "events", "relevance": "0.3178925934144105"}, {"concept": "effect", "relevance": "0.31472978633378823"}, {"concept": "purpose", "relevance": "0.29572991807651383"}]
|
|
Array of < Literal: String > |
List of medical conditions names, e.g. ‘Breast cancer’ or ‘Obesity’. |
["Cognitive Therapy", "Primary Prevention", "Caregivers", "Mental Health Wellness 1", "Adolescent - Emotional Problem"]
|
|
Literal: Timestamp |
Timestamp when the clinical trial was last imported into BigQuery. Incremental changes can be determined using this field. Note, however at certain times all records may be reimported. |
"2020-08-05 03:00:30 UTC"
|
|
Literal: Timestamp |
Date when the record was inserted into Dimensions. |
"2020-08-05 03:00:30 UTC"
|
|
Literal: String |
End date of a clinical trial. |
"2021-03"
|
|
Literal: Integer |
End year of the clinical trial. |
2021
|
|
Array of < Literal: String > |
GRID ID of organisations funding the clinical trial. |
["grid.239864.2", "grid.65499.37"]
|
|
Array of < Entity: Funding > |
List of details regarding grants associated to the clinical trial. |
[{"grid_id": "grid.419635.c", "grant_id": "grant.2690176"}, {"grid_id": "grid.419635.c", "grant_id": "grant.6443089"}, {"grid_id": "grid.239864.2", "grant_id": null}, {"grid_id": "grid.65499.37", "grant_id": null}]
|
|
Literal: String |
The gender of the clinical trial subjects e.g. ‘Male’, ‘Female’ or ‘All’. |
"All"
|
|
Literal: String |
Dimensions clinical trial ID. |
"NCT04114864"
|
|
Array of < Entity: Intervention > |
Information about the clinical trial’s interventions according to the research plan or protocol created by the investigators. |
[{"arm_group_labels": "Dulaglutide", "description": "Administered SC", "type": "Drug", "name": "Dulaglutide", "other_names": "LY2189265"}, {"arm_group_labels": "Tirzepatide", "description": "Administered SC", "type": "Drug", "name": "Tirzepatide", "other_names": "LY3298176"}]
|
|
Array of < Entity: Investigator > |
List of additional details regarding investigators, including affiliations and roles. |
[{"first_name": "Elizabeth", "last_name": "JHanson", "researcher_id": null, "affiliations": [{"affiliation": "Linneus University, Kalmar, Sweden", "grid_ids": [], "cities": [], "countries_territories": ["SE"], "states": []}]}, {"first_name": "Licia", "last_name": "Boccaletti", "researcher_id": "ur.014561655145.71", "affiliations": [{"affiliation": "Anziani e non solo soc. coop. soc", "grid_ids": [], "cities": ["3180445"], "countries_territories": ["IT"], "states": []}]}, {"first_name": "Frans", "last_name": "VanZoest", "researcher_id": null, "affiliations": [{"affiliation": "Stichting Vilans", "grid_ids": ["grid.438099.f"], "cities": ["2745912"], "countries_territories": ["NL"], "states": []}]}, {"first_name": "Valentina", "last_name": "Hlebec", "researcher_id": "ur.07551030232.17", "affiliations": [{"affiliation": "University of Ljubljana", "grid_ids": ["grid.8954.0"], "cities": ["3196359"], "countries_territories": ["SI"], "states": []}]}, {"first_name": "Agnes", "last_name": "Leu", "researcher_id": "ur.01352632125.37", "affiliations": [{"affiliation": "Stiftung Kalaidos Fachhochschule (Kalaidos FH)", "grid_ids": ["grid.449532.d"], "cities": ["2657896"], "countries_territories": ["CH"], "states": []}]}, {"first_name": "Eva", "last_name": "Jolly", "researcher_id": null, "affiliations": [{"affiliation": "Carers Trust, Print Rooms, 164-180 Union Street, London, SE1 0LN. Carers Trust will be co-ordinating the completion of the interventions for the clinical trials in the UK. All trials for the ME-WE project will be completed in England.", "grid_ids": ["grid.501037.0"], "cities": ["2643743"], "countries_territories": ["GB"], "states": []}]}]
|
|
Literal: String |
Original URL for the clinical trial. |
"https://clinicaltrials.gov/show/NCT04114864"
|
|
Array of < Literal: String > |
Medical Subject Heading terms as used in PubMed. |
["Nervous System Diseases", "Heredodegenerative Disorders, Nervous System", "Pathologic Processes", "Rett Syndrome", "Intellectual Disability", "Neurobehavioral Manifestations", "Syndrome", "Genetic Diseases, X-Linked", "Mental Retardation, X-Linked", "Neurologic Manifestations", "Disease", "Genetic Diseases, Inborn"]
|
|
Array of < Entity: Organisation > |
List of details regarding organisations involved, e.g. as sponsors or collaborators. |
[{"raw_name": "Valentina Hlebec", "grid_id": null}, {"raw_name": "Anziani e non solo soc. coop. soc", "grid_id": null}, {"raw_name": "Stichting Vilans", "grid_id": "grid.438099.f"}, {"raw_name": "University of Ljubljana", "grid_id": "grid.8954.0"}, {"raw_name": "Linnaeus University, Nationellt kompetenscentrum anh\u00f6riga (Nka), (Swedish Family Care Competence Centre)", "grid_id": "grid.8148.5"}, {"raw_name": "Stiftung Kalaidos Fachhochschule (Kalaidos FH)", "grid_id": "grid.449532.d"}, {"raw_name": "Carers Trust, Print Rooms, 164-180 Union Street, London, SE1 0LN. Carers Trust will be co-ordinating the completion of the interventions for the clinical trials in the UK. All trials for the ME-WE project will be completed in England.", "grid_id": "grid.501037.0"}, {"raw_name": "Linneus University, Kalmar, Sweden", "grid_id": null}, {"raw_name": "Anziani e non solo soc. coop. soc", "grid_id": null}, {"raw_name": "Stichting Vilans", "grid_id": "grid.438099.f"}, {"raw_name": "University of Ljubljana", "grid_id": "grid.8954.0"}, {"raw_name": "Stiftung Kalaidos Fachhochschule (Kalaidos FH)", "grid_id": "grid.449532.d"}, {"raw_name": "Carers Trust, Print Rooms, 164-180 Union Street, London, SE1 0LN. Carers Trust will be co-ordinating the completion of the interventions for the clinical trials in the UK. All trials for the ME-WE project will be completed in England.", "grid_id": "grid.501037.0"}]
|
|
Literal: String |
Status of the trial as provided by the registry. |
"Completed"
|
|
Literal: String |
Phase of the clinical trial, as a string. |
"Phase 1/2"
|
|
Literal: String |
Primary completion date provided by trial, year-month or just year. |
"2003-07"
|
|
Literal: Integer |
Primary completion year provided by trial. |
"2003"
|
|
Array of < Literal: String > |
List of Dimensions IDs of publications cited by the clinical trial. |
["pub.1045250304", "pub.1113522247", "pub.1047486020", "pub.1009955273", "pub.1040139682", "pub.1030908595", "pub.1004866948", "pub.1027730547", "pub.1020556064", "pub.1035229386", "pub.1015646550", "pub.1058370884", "pub.1058267639"]
|
|
Literal: Timestamp |
The date the trial is entered onto a publicly accessible register. |
|
|
Literal: String |
The year the trial is entered onto a publicly accessible register. |
|
|
Literal: String |
The platform where the clinical trial has been registered, e.g. ‘ClinicalTrials.gov’ or ‘EU-CTR’. |
"ClinicalTrials.gov"
|
|
Array of < Literal: String > |
GRID ID of organisations involved, e.g. as sponsors or collaborators. |
["grid.422126.1", "grid.12082.39", "grid.8148.5", "grid.438099.f", "grid.501037.0", "grid.8954.0", "grid.1214.6", "grid.449532.d"]
|
|
Array of < Literal: String > |
Dimensions researchers IDs associated to the clinical trial. |
["ur.07551030232.17", "ur.014561655145.71", "ur.01352632125.37"]
|
|
Array of < Literal: String > |
List of Dimensions IDs of publications resulting from the clinical trial. |
|
|
Literal: String |
The date on which summary results information was first available. |
|
|
Array of < Entity: SecondaryIdDetail > |
Details about additional identifier, other than the organization’s Unique Protocol Identification Number that is assigned to the clinical study. |
|
|
Array of < Literal: String > |
Identifiers, other than parent registry identification number, assigned to the study such as grant, contract numbers or an identification number assigned by another registry. |
["CDR0000065549", "NCCTG-934655"]
|
|
Literal: String |
Start date of a clinical trial. |
"2019-10"
|
|
Literal: Integer |
Start year of the clinical trial. |
2019
|
|
Array of < Entity: StudyArm > |
The groups or subgroups of participants that receives specific a set of interventions/treatments, or no intervention, according to the trial’s protocol. |
[{"type": "Experimental", "label": "LY3295668 Erbumine Escalation", "description": "LY3295668 Erbumine given orally."}, {"type": "Experimental", "label": "LY3295668 Erbumine + Topotecan + Cyclophosphamide Escalation", "description": "LY3295668 Erbumine given orally and topotecan and cyclophosphamide given intravenously (IV)."}, {"type": "Experimental", "label": "LY3295668 Erbumine Expansion", "description": "LY3295668 Erbumine given orally."}, {"type": "Experimental", "label": "LY3295668 Erbumine + Topotecan + Cyclophosphamide Expansion", "description": "LY3295668 Erbumine given orally and topotecan and cyclophosphamide given IV."}]
|
|
Array of < Entity: StudyDesign > |
Details regarding the design of a clinical trial. |
[{"allocation": "Randomized", "intervention_model": "Parallel Assignment", "primary_purpose": "Treatment", "masking": "None (Open Label)"}]
|
|
Literal: String |
Criteria for participation in the clinical study. |
"1. Relatives of patients;\n2. Aged >= 18 years;\n3. Is taking care of the patient, and will have a certain amount of time to take care of the patient every week for the next month (multiple caregivers, prefer the one with the longest care time);\n4. Recognizing that the patient's disease is incurable, the patient's survival is assessed by the attending physician according to KPS and other scales within six months, and not less than one month;\n5. Through the questionnaire evaluation, it is in a \" intentional state\";\n6. Good WeChat skills."
|
|
Literal: String |
Eligibility criteria for a person to not participate in clinical study. |
|
|
Literal: String |
Eligibility criteria for a person to participate in clinical study. |
|
|
Literal: String |
Maximum age eligible for the study. |
"35"
|
|
Literal: String |
Minimum age eligible for the study. |
"18"
|
|
Array of < Entity: OutcomeMeasure > |
List of measures of outcome associated to the clinical trial. |
[{"type": "primary", "measure": "Number of Participants with Dose Limiting Toxicities (DLTs)", "time_frame": "Baseline through Cycle 2 (28 Day Cycle)"}, {"type": "primary", "measure": "Overall Response Rate (ORR): Percentage of Participants Who Achieve Complete Response (CR) or Partial Response (PR)", "time_frame": "Baseline through Measured Progressive Disease (Estimated up to 5 Years)"}, {"type": "primary", "measure": "Duration of Response (DoR)", "time_frame": "Date of CR or PR to Date of Disease Progression or Death Due to Any Cause (Estimated up to 5 Years)"}, {"type": "secondary", "measure": "Pharmacokinetics (PK): Area Under the Concentration Time Curve (AUC) of LY3295668", "time_frame": "Cycle 1 Day 1 through Cycle 1 Day 15 (28 Day Cycles)"}, {"type": "secondary", "measure": "PK: AUC of LY3295668 in Combination with Topotecan and Cyclophosphamide", "time_frame": "Cycle 1 Day 1 through Cycle 1 Day 15 (28 Day Cycles)"}, {"type": "secondary", "measure": "Best Overall Response (BOR): Percentage of Participants with CR, PR, Stable Disease (SD), or Progressive Disease (PD)", "time_frame": "Baseline to Date of Objective Disease Progression (Estimated up to 5 Years)"}, {"type": "secondary", "measure": "Progression-Free Survival (PFS)", "time_frame": "Baseline to Objective Progression or Death Due to Any Cause (Estimated up to 5 Years)"}, {"type": "secondary", "measure": "Overall Survival (OS)", "time_frame": "Baseline to Date of Death from Any Cause (Estimated up to 6 Years)"}]
|
|
Literal: Integer |
The actual or estimated target number of participants. |
2500
|
|
Literal: String |
The type of study associated to the clinical trial e.g. ‘Interventional’, ‘Observational’, etc. |
"Interventional study"
|
|
Literal: String |
The title of the clinical trial. |
"A Primary Prevention Intervention for the Promotion of Psycho-social Wellbeing in Adolescent Young Carers: a Randomized Control Trial in the Project H2020 ME-WE"
|
Clinical Trials Links
The diagram below provides an overview of the outgoing links from Clinical Trials to other sources.
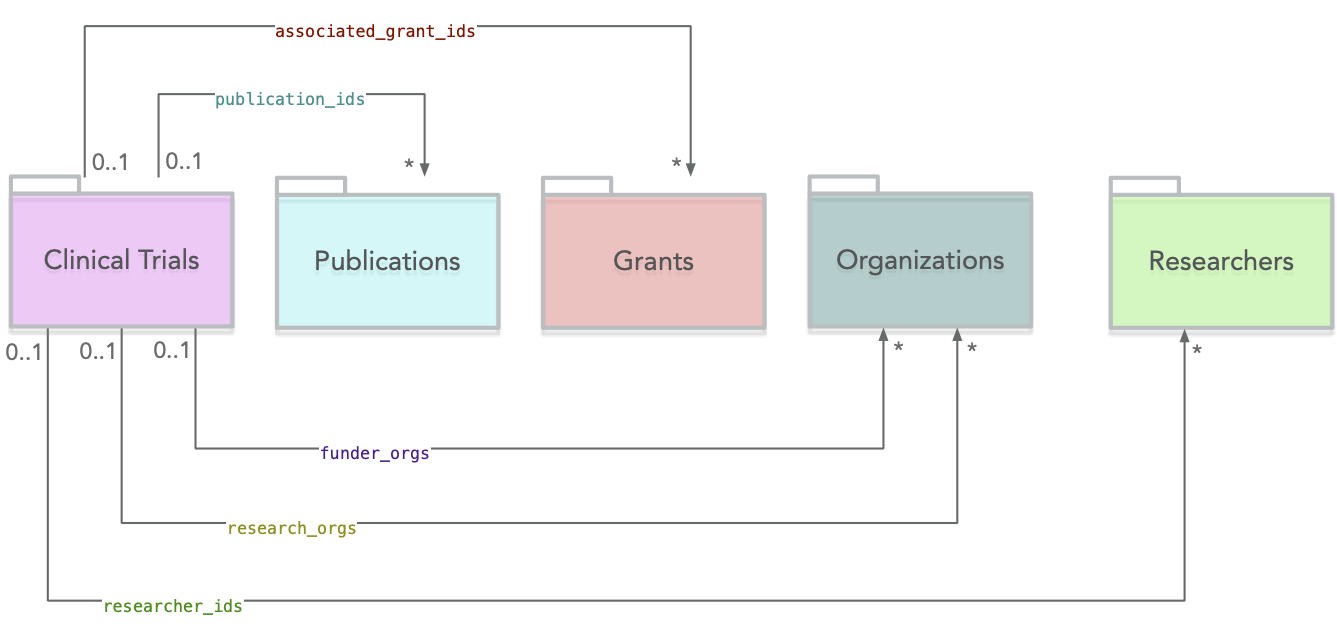
Auxiliary Entities
Affiliation
Field |
Type |
Description |
|---|---|---|
|
Literal: String |
The raw affiliation string as seen on the source type. |
|
Array of < Literal: Integer > |
The cities identified in the affiliation, as geoname IDs. |
|
Array of < Literal: String > |
The country/territories identified in the affiliation, as ISO-3166 alpha-2 codes. |
|
Array of < Literal: String > |
The assigned GRID IDs based on this affiliation. |
|
Array of < Literal: String > |
The states/regions identified in the affiliation, as ISO 3166-2 codes. |
Altmetric
Field |
Type |
Description |
|---|---|---|
|
Literal: String |
Altmetrics record identifier |
|
Literal: Integer |
ComplexSystematicCategorization
Field |
Type |
Description |
|---|---|---|
|
Entity: SystematicCategoryLevel |
First level of categorisation within the classification scheme. |
|
Entity: SystematicCategoryLevel |
Second level of categorisation within the classification scheme. |
Concept
Field |
Type |
Description |
|---|---|---|
|
Literal: String |
Normalized noun phrase describing a main topic within the document. Automatically derived using machine learning techniques. |
|
Literal: Float |
Relevance ranking of the normalized noun phrase within the document’s field of study. |
Funding
Field |
Type |
Description |
|---|---|---|
|
Literal: String |
The Dimensions grant ID, if identified. |
|
Literal: String |
The GRID ID of the funder, if identified. |
Intervention
Field |
Type |
Description |
|---|---|---|
|
Literal: String |
|
|
Literal: String |
|
|
Literal: String |
|
|
Literal: String |
|
|
Literal: String |
Investigator
Field |
Type |
Description |
|---|---|---|
|
Array of < Entity: Affiliation > |
Affiliation details associated with the clinical investigator. |
|
Literal: String |
|
|
Literal: String |
|
|
Literal: String |
The Dimensions assigned researcher ID. |
|
Literal: String |
Role of the clinical investigator (ie. Principal Investigator, Study Chair/Director, Contact). |
Organisation
Field |
Type |
Description |
|---|---|---|
|
Array of < Literal: Integer > |
The cities identified in the affiliation, as GeoName identifiers. |
|
Array of < Literal: String > |
Country/territories identified in the affiliation, as ISO-3166 alpha-2 codes. |
|
Literal: String |
The resolved GRID ID of the organisation. |
|
Literal: String |
Type of the organisation involved, collected from the clinical trial registry. |
|
Literal: String |
The organisation name as collected from the clinical trial registry. |
|
Array of < Literal: String > |
The states/regions identified in the affiliation, as ISO 3166-2 codes. |
|
Literal: String |
The type of organisation associated with the clinical trial (sponsor, collaborator, facility or affiliated). |
OutcomeMeasure
Field |
Type |
Description |
|---|---|---|
|
Literal: String |
Description of the measure. |
|
Literal: String |
Details of the timeframe related to the measure. |
|
Literal: String |
Type assigned to the outcome measure e.g. ‘primary’, ‘secondary’, etc. |
SecondaryIdDetail
Field |
Type |
Description |
|---|---|---|
|
Literal: String |
Name or brief description of organization that issued the identifier. |
|
Literal: String |
An identifier(s) (ID), if any, other than the organization’s Unique Protocol Identification Number that is assigned to the clinical study. |
|
Literal: String |
A brief description of the type of identifier. |
SimpleCategorization
Field |
Type |
Description |
|---|---|---|
|
Array of < Entity: SimpleCategory > |
A complete listing of category values, assigned to this particular record, including the unique Dimensions identifier associated with each category value. |
|
Array of < Literal: String > |
Listing of all category values assigned to this particular record. |
SimpleCategory
Field |
Type |
Description |
|---|---|---|
|
Literal: String |
Dimensions specific identifier assigned to the category value. |
|
Literal: String |
Category value associated with the simple classification scheme. |
StudyArm
Field |
Type |
Description |
|---|---|---|
|
Literal: String |
Description of the study arm. |
|
Literal: String |
Label assigned to the study arm. |
|
Literal: String |
Type of the study arm e.g. ‘Active Comparator’, ‘Experimental’, ‘No intervention’, etc. |
StudyDesign
Field |
Type |
Description |
|---|---|---|
|
Literal: String |
|
|
Literal: String |
|
|
Literal: String |
|
|
Literal: String |
SystematicCategorization
Field |
Type |
Description |
|---|---|---|
|
Array of < Literal: String > |
Listing of all category specific codes assigned to this particular record. |
|
Array of < Entity: SystematicCategory > |
A complete listing of category values, assigned to this particular record, including the unique Dimensions identifier associated with each category value. |
SystematicCategory
Field |
Type |
Description |
|---|---|---|
|
Literal: String |
Numeric or non-numeric category value code. Value is classification scheme specific. |
|
Literal: String |
Dimensions specific identifier assigned to the category value. |
|
Literal: String |
Individual category value description, excluding the classification scheme specific code prefix. |
SystematicCategoryLevel
Field |
Type |
Description |
|---|---|---|
|
Array of < Literal: String > |
A consolidated list of all category-specific codes at a particular classification level, assigned to this record. |
|
Array of < Entity: SystematicCategory > |
A listing of category values at a specific classification level, assigned to this particular record, including the unique Dimensions identifier associated with each category value. |
VersionedCategories
Field |
Type |
Description |
|---|---|---|
|
Entity: SimpleCategorization |
|
|
Entity: ComplexSystematicCategorization |
|
|
Entity: SimpleCategorization |
|
|
Entity: SimpleCategorization |
|
|
Entity: SystematicCategorization |
|
|
Entity: SystematicCategorization |
|
|
Entity: SimpleCategorization |
|
|
Entity: SimpleCategorization |
|
|
Entity: SimpleCategorization |
Clinical Trials Deprecations
This section lists fields that have been deprecated and should no longer be used within queries. In time, deprecated fields will be removed from the data source’s corresponding schema in BigQuery.
Deprecated Fields
Field |
Type |
Description |
|---|---|---|
|
Entity: SystematicCategorization |
Deprecated with no recommended replacement. |
|
Entity: SystematicCategorization |
Deprecated with no recommended replacement. |